ni valence electrons|Iba pa : Pilipinas Learn how to determine the number of valence electrons for an element using the periodic table. See patterns, examples, and tips for main group and transition metals.
Cinema movie schedule in CityMall Boracay. Cinema movie schedule in CityMall Boracay. Movies TV Food & Drink Shops & Services. Tickets Menu. Get Tickets. Ayala Malls Cinemas Robinsons Movieworld . Awaiting schedule for today, Thursday, September 5, 2024. You Might Also Like.
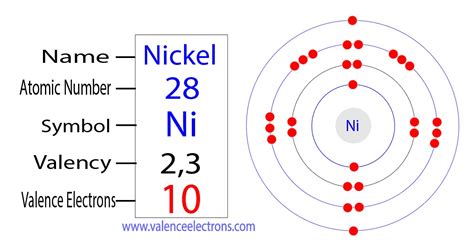
ni valence electrons,Mar 23, 2023 Ni – 2e – → Ni 2+. Here, the electron configuration of nickel ion (Ni 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 8. This electron configuration shows that nickel ion (Ni 2+) has three shells and the last .

To find the number of valence electrons for Nickel (Ni) we need to look at its electron configuration. This is necessary because Ni is a transition metal (d block element) and we need to take.
Valence shell. The valence shell is the outermost shell of an atom in its uncombined state, which contains the electrons most likely to account for the nature of any reactions involving the atom and of the . Here we have covered the Nickel Valence Electrons and Nickel Valency (Ni) with Dot Diagram. Other infomation about Nickel also covered.Learn how to determine the number of valence electrons for an element using the periodic table. See patterns, examples, and tips for main group and transition metals. Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as .Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) sublevel are called . Valence electrons are outer shell electrons for main group elements. For the transition metals with partially-filed d shells, valence electrons are those electrons outside the noble gas core. The .Iba pa Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the \(1s\) . It uses the representative symbol of Nickel (Ni) to draw the valence electrons around it. So, the numbers of dots show are exactly the numbers of valence electrons. You can draw the Lewis dot diagram for any chemical element. Valency of Nickel. The valency or the combining capacity of Nickel is 1,2, 3,4. It has the variable . The valence shell is the outermost shell of an atom in its uncombined state, which contains the electrons most likely to account for the nature of any reactions involving the atom and of the bonding .ni valence electrons The electronic configuration is defined as the number of electrons distributed in the atom’s or molecule’s orbits. Nickel has 28 electrons which are distributed among the 4 orbits of its atom. .ni valence electrons Iba paHow to Determine Number of Valence Electrons in Element, Ion, and Compound (Count Valence Electrons) Conquer Chemistry. 742. views. 06:54. Valence Electrons. Duell Chemistry. 373. views. 05:29. How to Find the Number of Valence Electrons for Transition Metals. Wayne Breslyn. 1562. views. 03:32. Valence Electrons Periodic Table. 3.1: Valence Electrons is shared under a CC BY-NC license and was authored, remixed, and/or curated by LibreTexts. Valence electrons are the electrons in the highest occupied principal energy level of an atom. In the second period elements, the two electrons in the 1s sublevel are called inner-shell electrons ..An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can . In this example, the electron configuration for Ni 2 + still kept its 3d 8, but lost the 4s 2 (became 4s 0) because the s-orbital has the highest energy level of n = 4 in this case. Therefore, the s-orbital will lose its electrons first, before the d-orbital, and so Ni 2+ can be written as [Ar] 4s 0 3d 8 OR [Ar] 3d 8.

You may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple.
ni valence electrons|Iba pa
PH0 · valence electrons chart
PH1 · valence electron configuration calculator
PH2 · periodic table valence electrons
PH3 · ni valence electron count
PH4 · list of valence electrons for each element
PH5 · how to find valence electrons
PH6 · how many electrons in nickel
PH7 · full electron configuration of nickel
PH8 · Iba pa